0631-5768777
0631-5674466
whkangzheng@163.com
Skin surface stitcher
Product Name: Disposable Skin Closure Device
Registrant: WEIHAI KANGZHENG MEDICAL EQUIPMENTS TECHNOLOGY CO.,LTD
Management category: Class II medical devices
Product Model: KFH50、KFH65、KFH80、KFH95、KFH110、KFH125、KFH140、KFH155、KFH170、KFH185、KFH200、KFH215、KFH230、KFH245、KFH260、KFH275、KFH290、KFH410、KFH445、KFH500
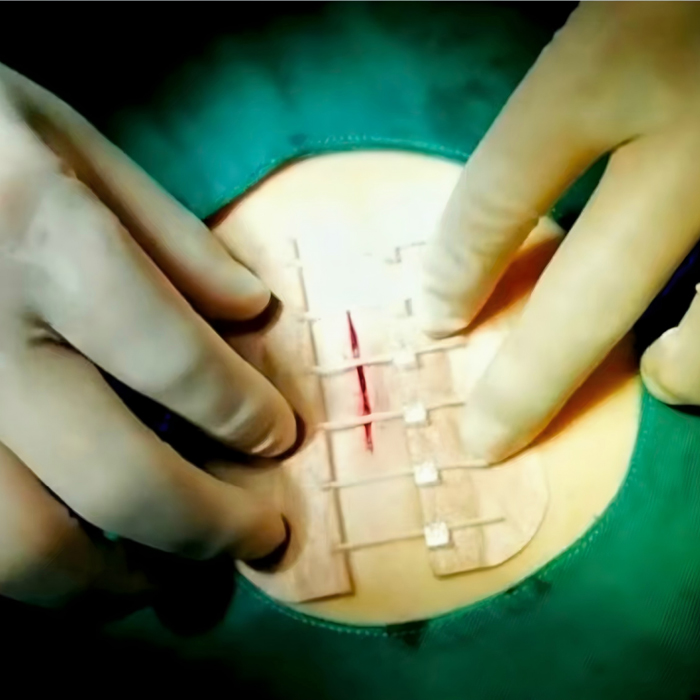
Product Structure: Consists of acrylic medical adhesive tape and a self-locking mechanism connecting the two tapes; the self-locking mechanism consists of a gear holder, gear, and rack.
Acrylic medical adhesive tape: Adhesive to the skin on both sides of the incision, providing adhesion.
Gear: Mated with the rack, it forms the operating element of the device.
Gear holder: Secures and supports the gear and features a spring retainer, limiting gear rotation to one direction, thereby enabling the rack to move in one direction.
Rack: Attached to the other side of the acrylic medical adhesive tape, pulling the rack closes the wound, achieving skin closure.
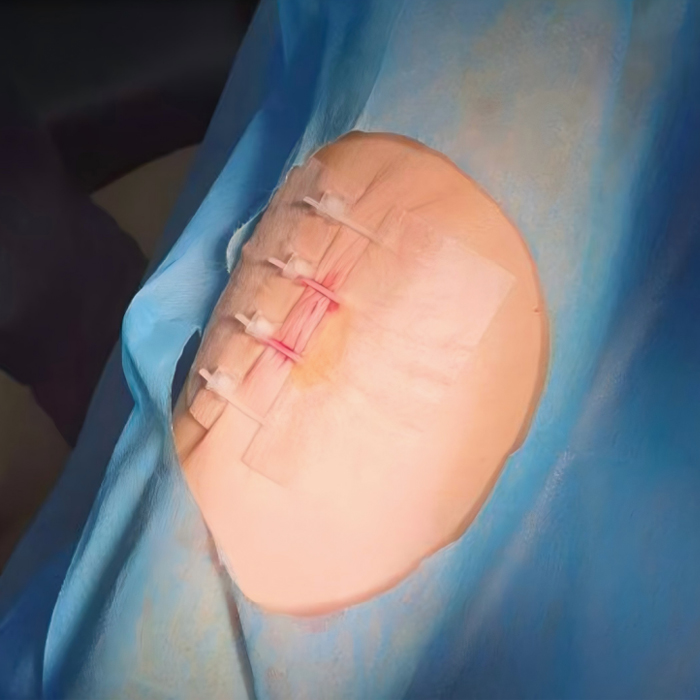
Product Features: This new surface suturing device utilizes the skin's own tension to perform non-invasive sutures on the skin surface. It offers advantages such as scar-free, minimal inflammatory response, aesthetically pleasing healing, no need for suture removal, and ease of use. It features high strength, microporous breathability, and hypoallergenic properties.
Adjusting the length of the suturing rack provides the desired suture tension for wound closure, achieving non-invasive, open, and tension-reducing suturing of incisions.
Skin Stapler: The integrated design ensures overall skin tension reduction on both sides of the incision; the parallel design ensures continuous, parallel alignment of the skin edges.
Self-locking mechanism: The built-in rotating gear ensures easy and effortless operation.
Rack: The thickened design perpendicular to the incision ensures smooth healing on both sides of the incision; the smooth bottom surface protects the skin surface from vectorial cutting and pressure.
Adhesive tape: 3M imported adhesive tape, tested by a national medical device testing center, is non-cytotoxic and non-allergenic.
Working principle: The inward pulling force of the tension reducer forms a uniform counterforce with the outward tension on both sides of the wound, thereby achieving the purpose of non-invasive suture-free, tension-reducing and closing, and preventing the wound from widening.
Scope of Application: Widely used in obstetrics, thoracic surgery, orthopedics, general surgery, urology, pediatric surgery, laparoscopic surgery, cosmetic surgery, and dermatology clinics.
Applicable Areas: Suitable for skin suturing on most areas of the body.
Intended Population: Patients requiring surgical procedures whose skin conditions are suitable for suturing with a stapler (specific determination to be made by a professional physician).
Intended Environment: For use by qualified medical personnel in qualified medical institutions (such as hospital operating rooms, emergency departments, and clinics).
Intended use: This product is suitable for use in surgical operations to replace traditional manual suturing and suture the human epidermis.
Contraindications:
1. Do not use if packaging is damaged;
2. For single use only; dispose of after use.
3. The production batch number, production date, and expiration date are visible on the seal;
4. The product is sterilized with ethylene oxide, and the sterility period is two years. Do not use the product after the expiration date.
5. This product is not suitable for critically ill patients, patients with functional failure, patients with severely contaminated wounds, or patients with severe malnutrition.
6. Do not use if you are allergic to this product.
7. This product should be used with caution if there is significant skin tissue loss.
Precautions for use
1. Professional Use: This product must be used by a trained physician who is familiar with its operation.
2. Single Use Only: This product is for single use only. Re-sterilization and reuse are strictly prohibited. Dispose of in accordance with medical waste disposal regulations after use.
3. Preoperative assessment: Before use, the doctor must assess the patient's tissue condition and whether the surgical site is suitable for the use of a suture device.
Please read the product instructions carefully or purchase and use under the guidance of medical personnel.
For contraindications or precautions, please refer to the instruction manual.



